Reflections
Reflections
Balancing vision and MVP
Need to smartly balance the product vision and feasible MVP scope given organisational ability and stakeholder risk appetite.
Simple structures all can follow
Keeping the decision-log simple and recording key approvals helps all team members secure the necessary approvals without delay
Simple structures all can follow
Keeping the decision-log simple and recording key approvals helps all team members secure the necessary approvals without delay
Product success comes from the depth of adoption not scale
Large scale adoption is not always the primary focus, especially when there are expert users in the mix
View into the process
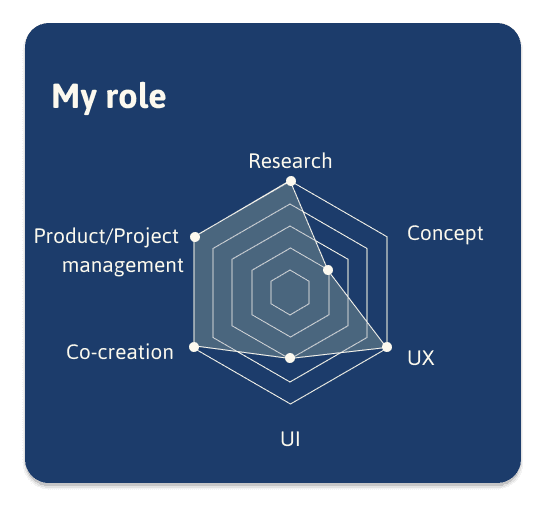


Reflections
Balancing vision and MVP
Need to smartly balance the product vision and feasible MVP scope given organisational ability and stakeholder risk appetite.
Simple structures all can follow
Keeping the decision-log simple and recording key approvals helps all team members secure the necessary approvals without delay
Product success comes from the depth of adoption not scale
Large scale adoption is not always the primary focus, especially when there are expert users in the mix

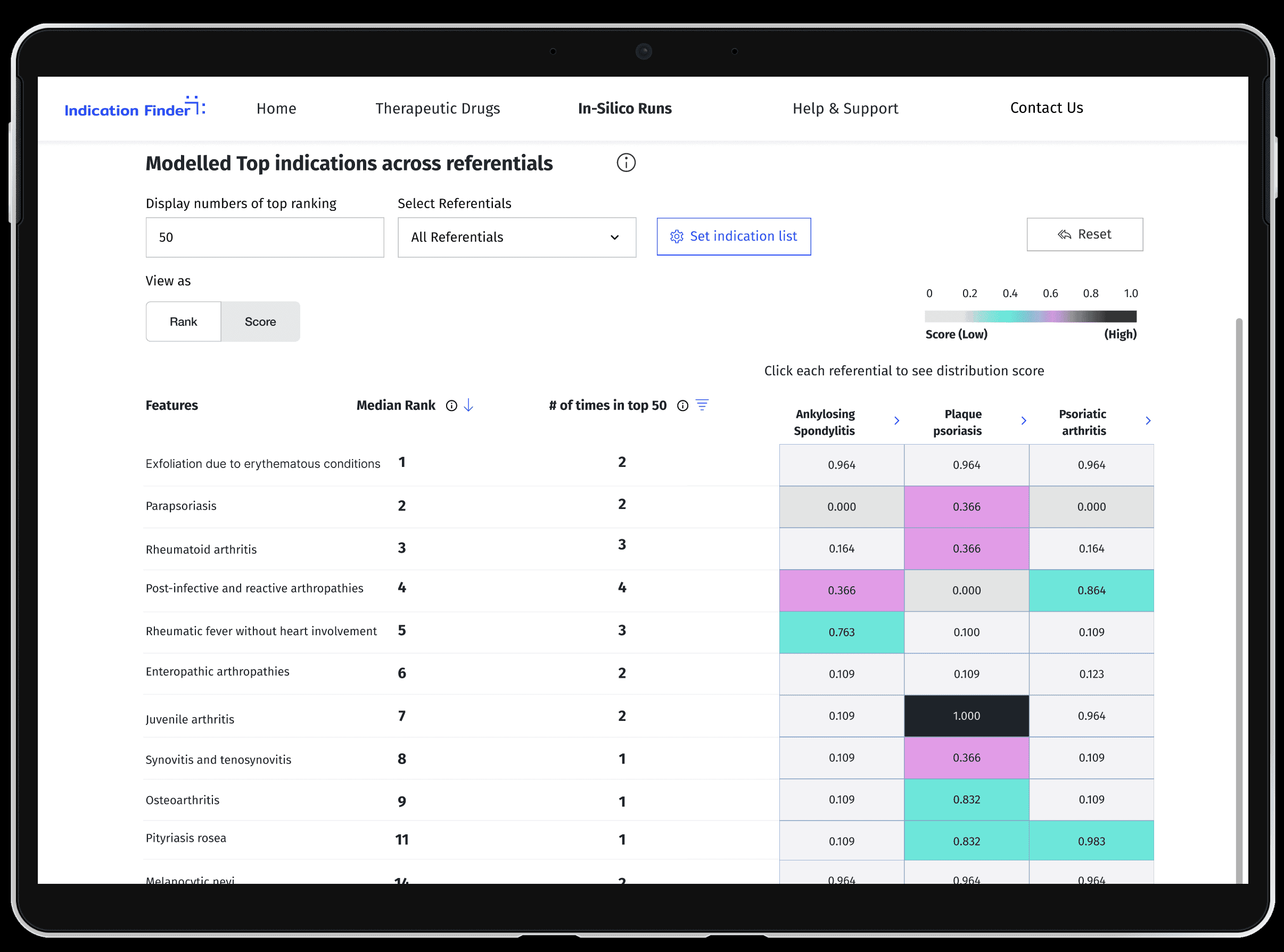

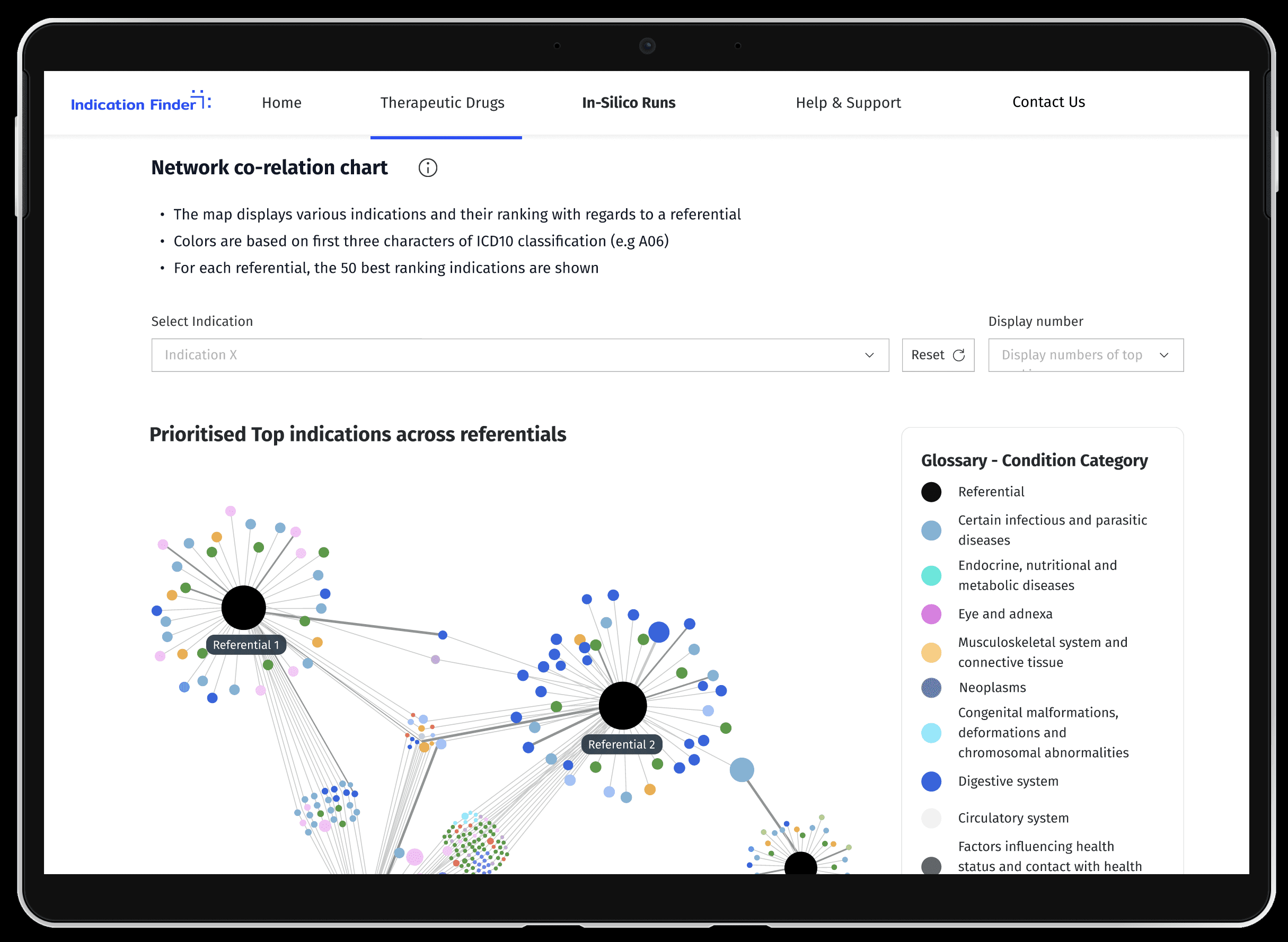














Context & Impact
Context
A PharmaCo developed AI-enabled indication finding capability, scaled to 10 immunology and neurology asset pipelines, resulting in a 2pp PTRS increase.
But the current process was time-consuming, un-automated, and challenged decision-making within different therapeutic areas.
Using rich text features within Confluence to conduct expert interviews and document initial takeaways that bring stakeholders along.
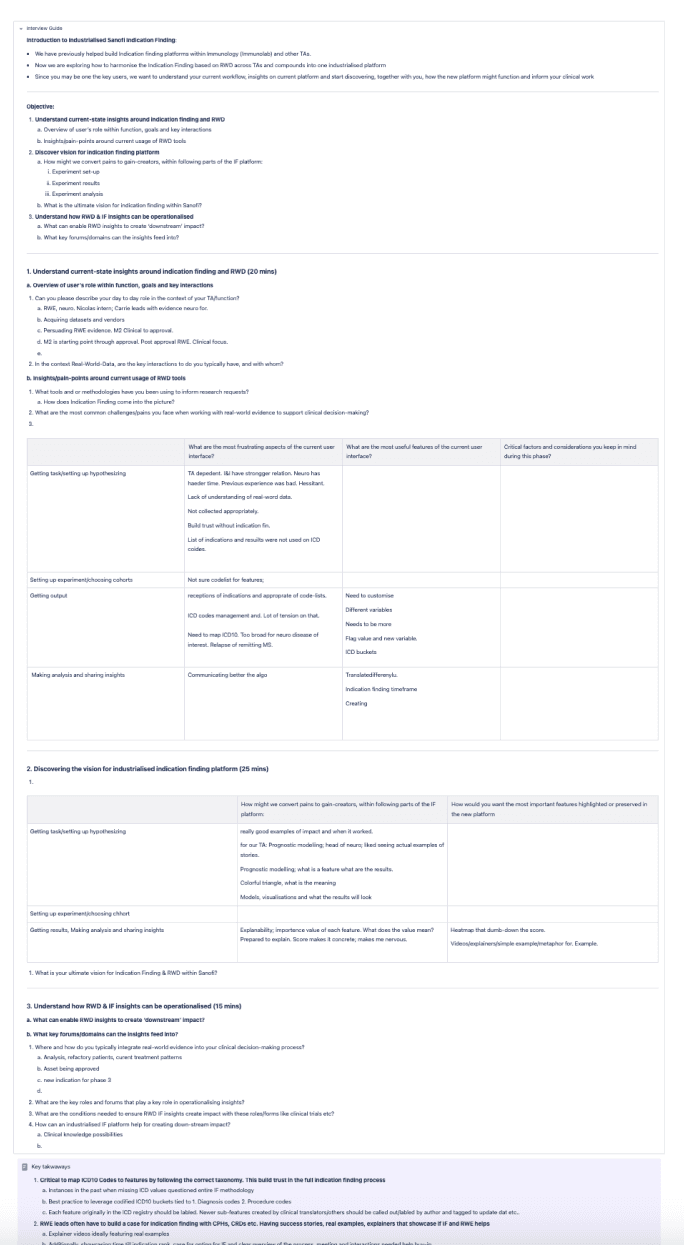
Expert interviews within guides with quick takeaways
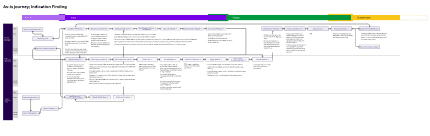
Understand IF workflow
Industrialised workflow
Operationalisation
Before making persona overviews, mapping all stakeholders (core, operational and enablers) and how they interact using the product we build.
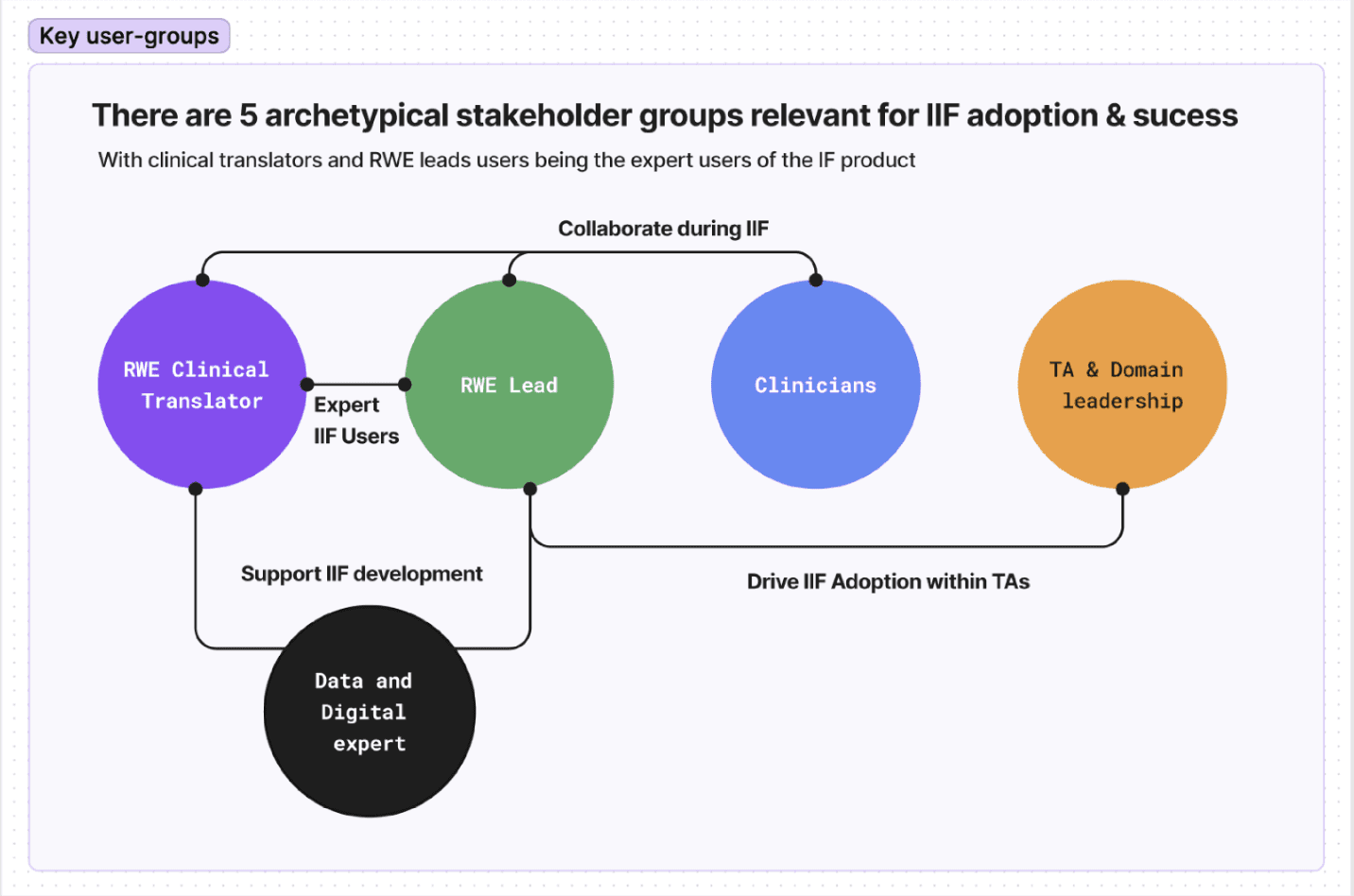
Understanding interactions and dependencies between roles
Understand IF workflow
Industrialised workflow
Operationalisation
Manual tasks, front-end tasks and back-end engineering tasks conducted pre-industrialisation, all documented with current-state insights and pain-points.
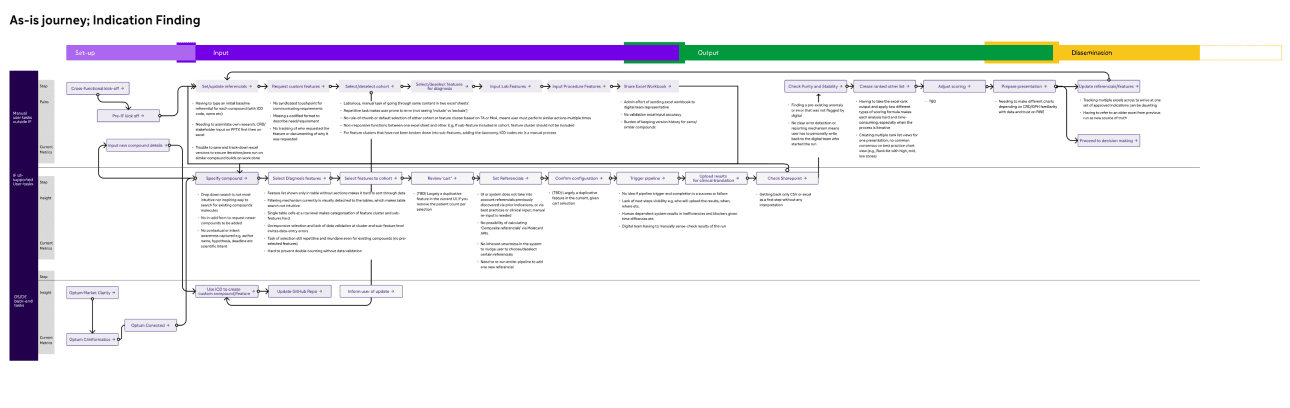
Documenting current workflows and handovers
Understand IF workflow
Industrialised workflow
Operationalisation
Reimagining a less manual and more automated front-end workflow and detailing features that address expert user’s needs.
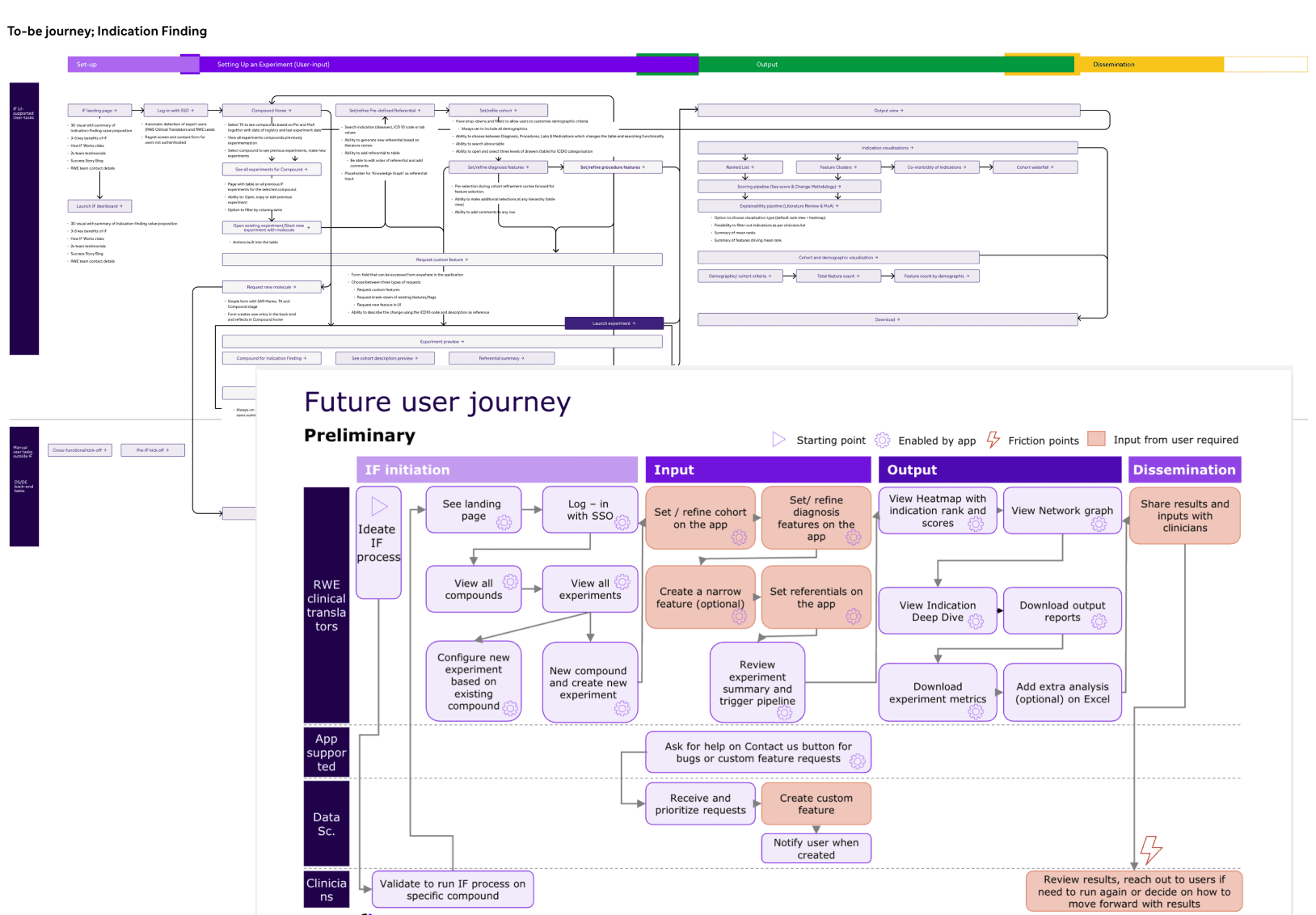
Anchoring product features in the future-state journey
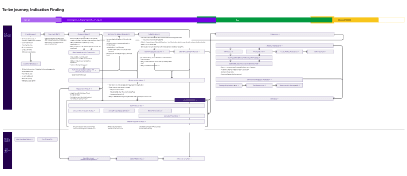
Understand IF workflow
Industrialised workflow
Operationalisation
Using design file as a foundation for early stage validation of interaction patterns and features.
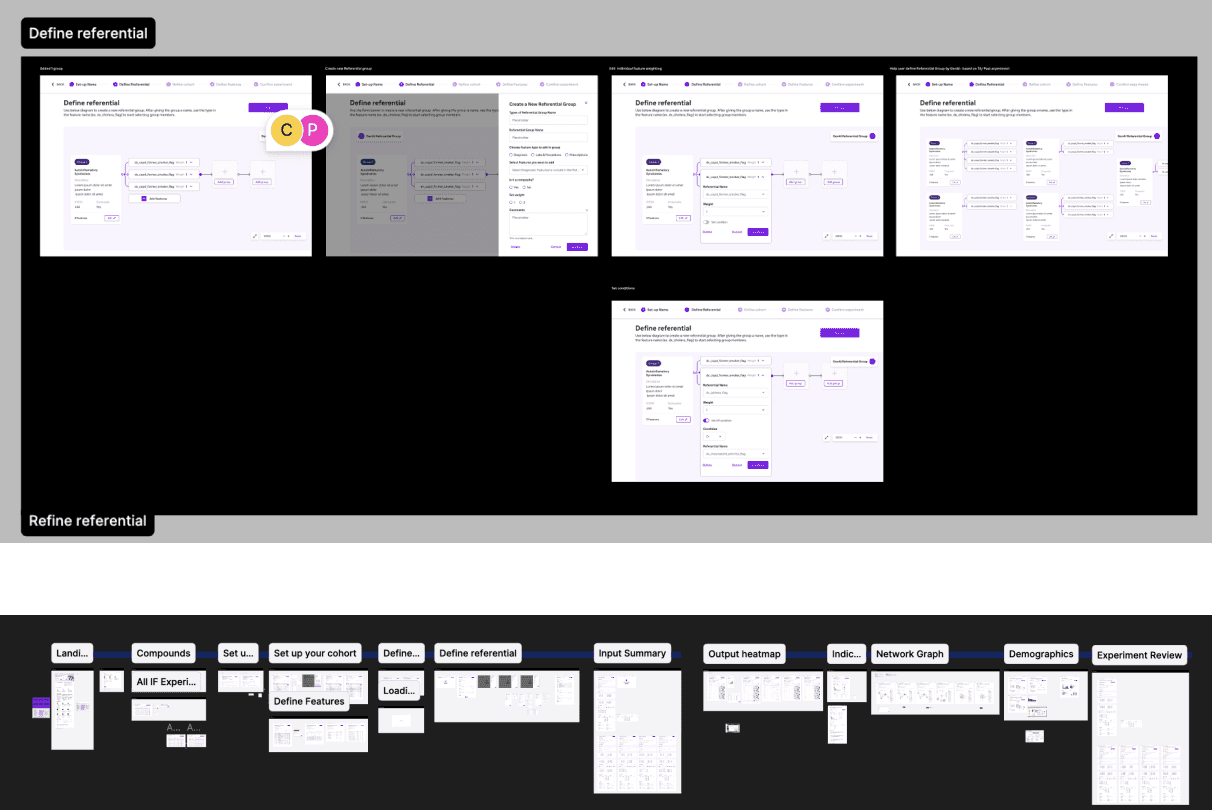
Iterative deployment via UX/UI prototyping
Understand IF workflow
Industrialised workflow
Operationalisation
Translating approved features within defined EPICS and detailed user-stories and sprint planning appropriate effort
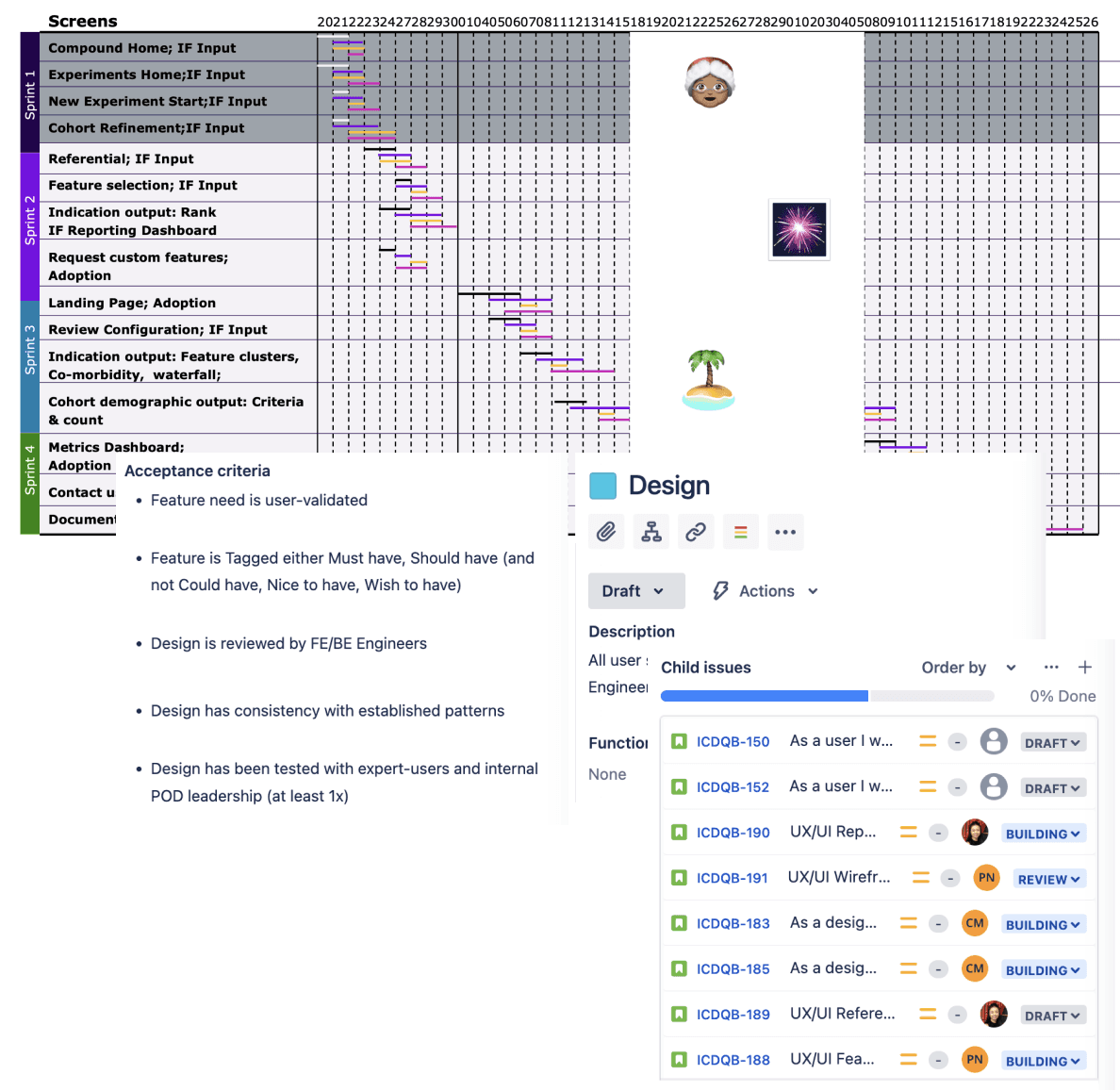
Playing product owner to translate requirements to build
Understand IF workflow
Industrialised workflow
Operationalisation
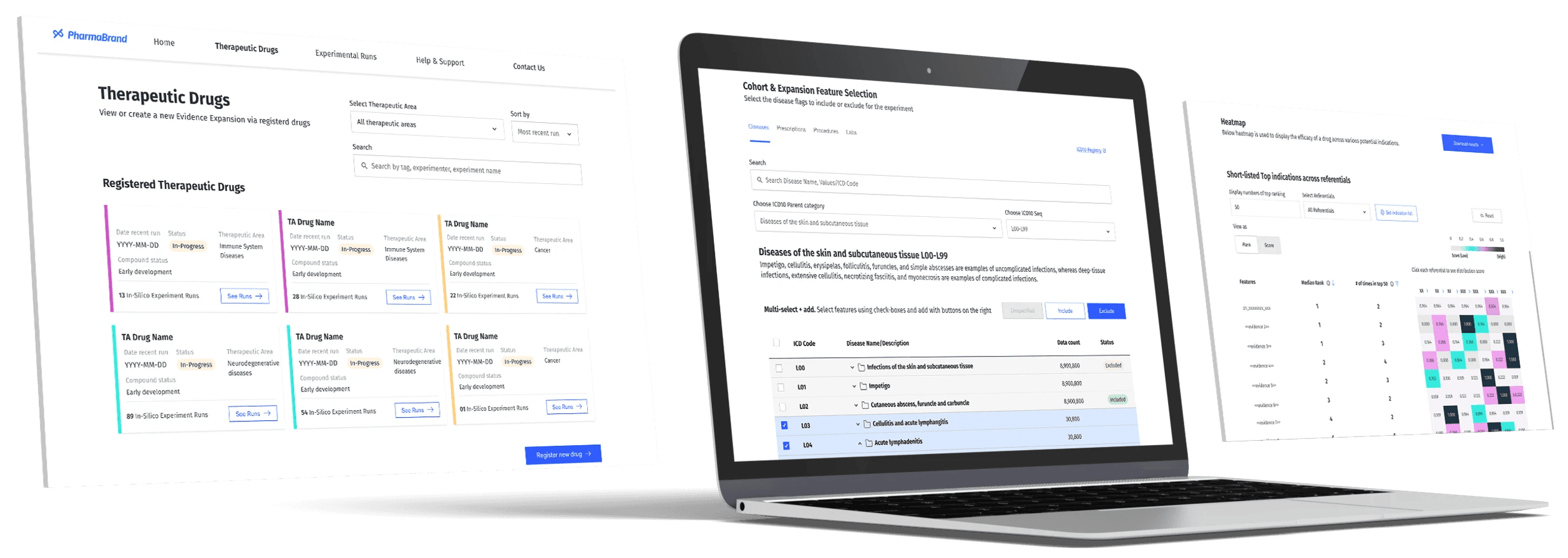
Factoring in the pharmaco asset pipeline to anticipate when the platform will be used most across therapeutic areas.
Designing platform adoption in line with pipeline
Understand IF workflow
Industrialised workflow
Operationalisation
Making the small tribe of clinical translators visible within the organisational intranet and showcasing their abilities.
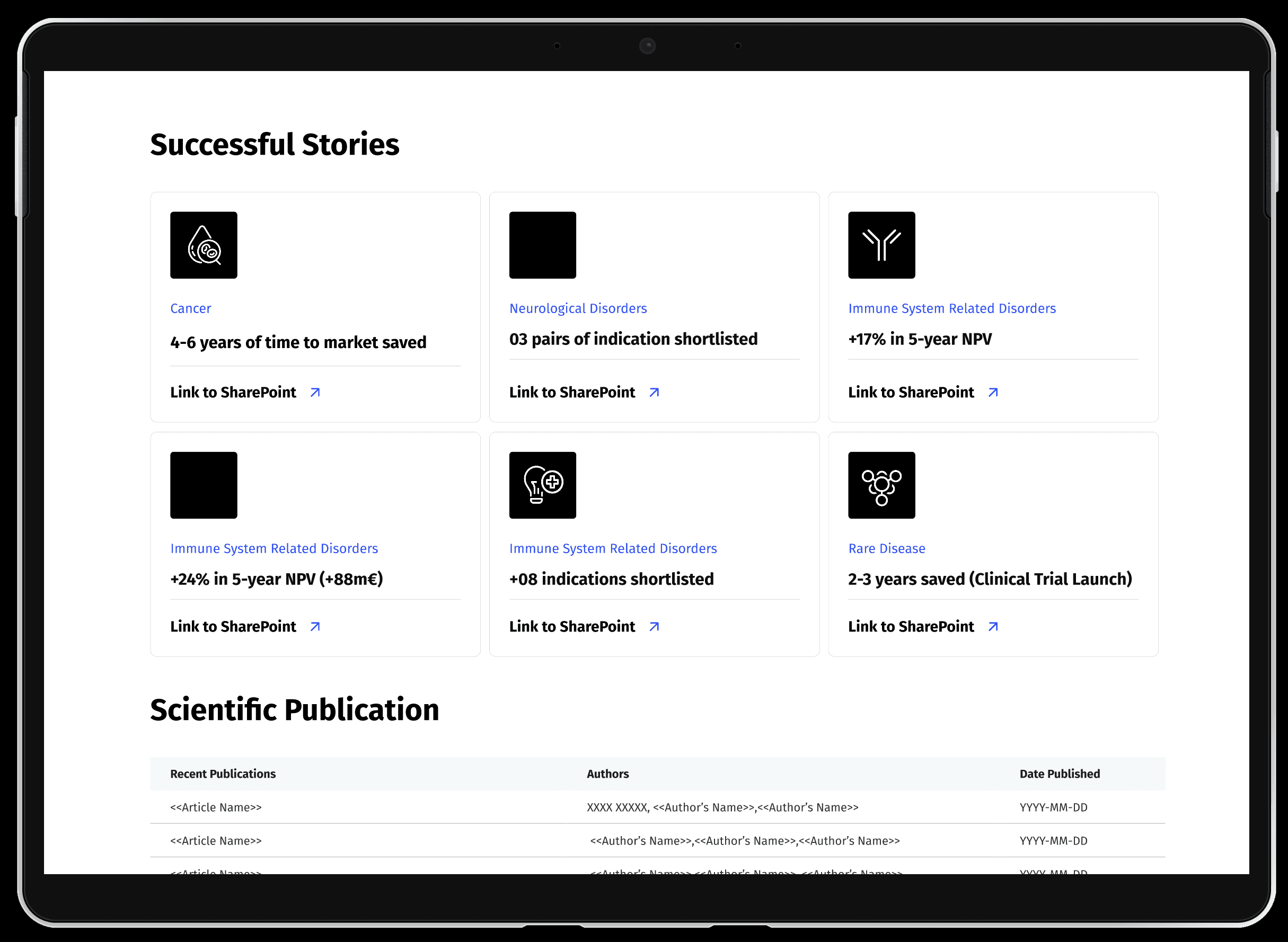
Showcasing capabilities via internal platform
Understand IF workflow
Industrialised workflow
Operationalisation
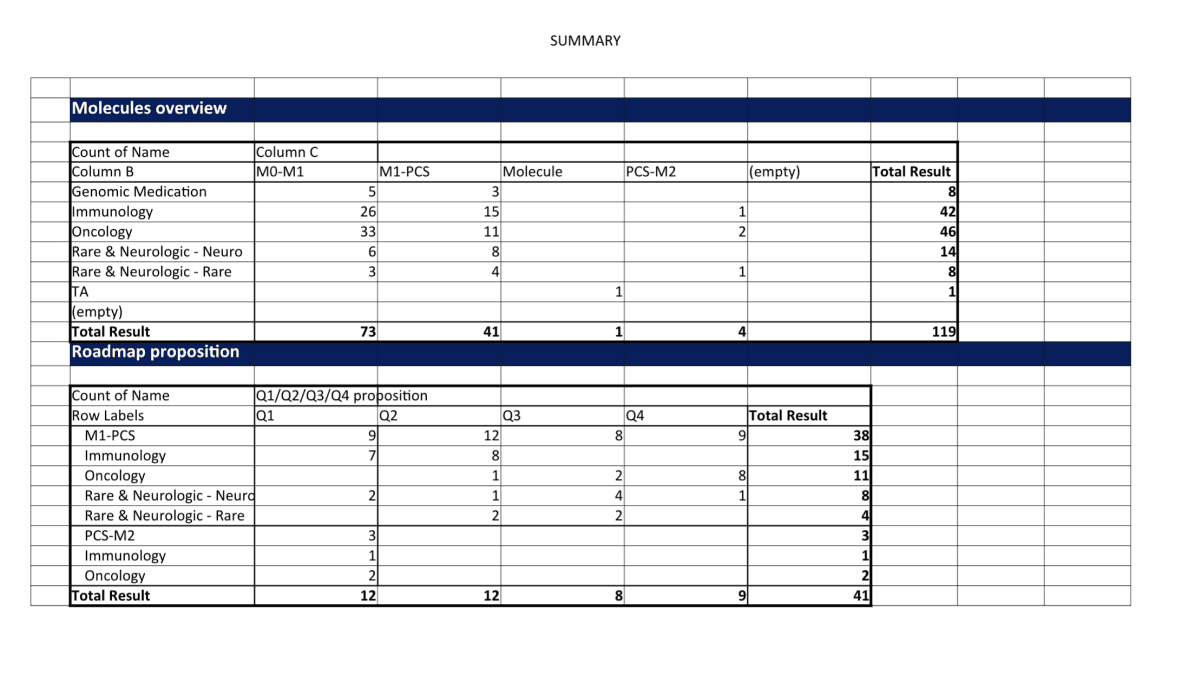
Harvesting user and tech requirements into a product roadmap and backlog of stories to be implemented in next quarters.
Documenting product roadmap with new EPICS
Understand IF workflow
Industrialised workflow
Operationalisation
Using rich text features within Confluence to conduct expert interviews and document initial takeaways that bring stakeholders along.

Expert interviews within guides with quick takeaways
Understand IF workflow
Industrialised workflow
Operationalisation
Before making persona overviews, mapping all stakeholders (core, operational and enablers) and how they interact using the product we build.

Understanding interactions and dependencies between roles
Understand IF workflow
Industrialised workflow
Operationalisation
Manual tasks, front-end tasks and back-end engineering tasks conducted pre-industrialisation, all documented with current-state insights and pain-points.

Documenting current workflows and handovers
Understand IF workflow
Industrialised workflow
Operationalisation
Reimagining a less manual and more automated front-end workflow and detailing features that address expert user’s needs.

Anchoring product features in the future-state journey
Understand IF workflow
Industrialised workflow
Operationalisation
Using design file as a foundation for early stage validation of interaction patterns and features.

Iterative deployment via UX/UI prototyping
Understand IF workflow
Industrialised workflow
Operationalisation
Translating approved features within defined EPICS and detailed user-stories and sprint planning appropriate effort

Playing product owner to translate requirements to build
Understand IF workflow
Industrialised workflow
Operationalisation
Factoring in the pharmaco asset pipeline to anticipate when the platform will be used most across therapeutic areas.

Designing platform adoption in line with pipeline
Understand IF workflow
Industrialised workflow
Operationalisation
Making the small tribe of clinical translators visible within the organisational intranet and showcasing their abilities.

Showcasing capabilities via internal platform
Understand IF workflow
Industrialised workflow
Operationalisation
Harvesting user and tech requirements into a product roadmap and backlog of stories to be implemented in next quarters.

Documenting product roadmap with new EPICS
Understand IF workflow
Industrialised workflow
Operationalisation
Using rich text features within Confluence to conduct expert interviews and document initial takeaways that bring stakeholders along.

Expert interviews within guides with quick takeaways
Understand IF workflow
Industrialised workflow
Operationalisation
Before making persona overviews, mapping all stakeholders (core, operational and enablers) and how they interact using the product we build.

Understanding interactions and dependencies between roles
Understand IF workflow
Industrialised workflow
Operationalisation
Manual tasks, front-end tasks and back-end engineering tasks conducted pre-industrialisation, all documented with current-state insights and pain-points.

Documenting current workflows and handovers
Understand IF workflow
Industrialised workflow
Operationalisation
Reimagining a less manual and more automated front-end workflow and detailing features that address expert user’s needs.

Anchoring product features in the future-state journey
Understand IF workflow
Industrialised workflow
Operationalisation
Using design file as a foundation for early stage validation of interaction patterns and features.

Iterative deployment via UX/UI prototyping
Understand IF workflow
Industrialised workflow
Operationalisation
Translating approved features within defined EPICS and detailed user-stories and sprint planning appropriate effort

Playing product owner to translate requirements to build
Understand IF workflow
Industrialised workflow
Operationalisation
Factoring in the pharmaco asset pipeline to anticipate when the platform will be used most across therapeutic areas.

Designing platform adoption in line with pipeline
Understand IF workflow
Industrialised workflow
Operationalisation
Making the small tribe of clinical translators visible within the organisational intranet and showcasing their abilities.

Showcasing capabilities via internal platform
Understand IF workflow
Industrialised workflow
Operationalisation
Harvesting user and tech requirements into a product roadmap and backlog of stories to be implemented in next quarters.

Documenting product roadmap with new EPICS
Understand IF workflow
Industrialised workflow
Operationalisation
Using rich text features within Confluence to conduct expert interviews and document initial takeaways that bring stakeholders along.

Expert interviews within guides with quick takeaways
Understand IF workflow
Industrialised workflow
Operationalisation
Before making persona overviews, mapping all stakeholders (core, operational and enablers) and how they interact using the product we build.

Understanding interactions and dependencies between roles
Understand IF workflow
Industrialised workflow
Operationalisation
Manual tasks, front-end tasks and back-end engineering tasks conducted pre-industrialisation, all documented with current-state insights and pain-points.

Documenting current workflows and handovers
Understand IF workflow
Industrialised workflow
Operationalisation
Reimagining a less manual and more automated front-end workflow and detailing features that address expert user’s needs.

Anchoring product features in the future-state journey
Understand IF workflow
Industrialised workflow
Operationalisation
Using design file as a foundation for early stage validation of interaction patterns and features.

Iterative deployment via UX/UI prototyping
Understand IF workflow
Industrialised workflow
Operationalisation
Translating approved features within defined EPICS and detailed user-stories and sprint planning appropriate effort

Playing product owner to translate requirements to build
Understand IF workflow
Industrialised workflow
Operationalisation
Factoring in the pharmaco asset pipeline to anticipate when the platform will be used most across therapeutic areas.

Designing platform adoption in line with pipeline
Understand IF workflow
Industrialised workflow
Operationalisation
Making the small tribe of clinical translators visible within the organisational intranet and showcasing their abilities.

Showcasing capabilities via internal platform
Understand IF workflow
Industrialised workflow
Operationalisation
Harvesting user and tech requirements into a product roadmap and backlog of stories to be implemented in next quarters.

Documenting product roadmap with new EPICS
Understand IF workflow
Industrialised workflow
Operationalisation
Impact
Faster time to go from input to meaningful output due to redesigned flows
∼7200%
In manual efforts from digital and data team for platform and pipeline topics
90% reduction
Collaboration between power users and medical and clinical stakeholders
Streamlined
Co-creative sessions with 7x power users over 8 weeks of build.
40+
To go from initial discovery to validated screen for React.JS FE and BE build
2-weeks
Additional clinical translation capacity unlocked via industrialised build
21x
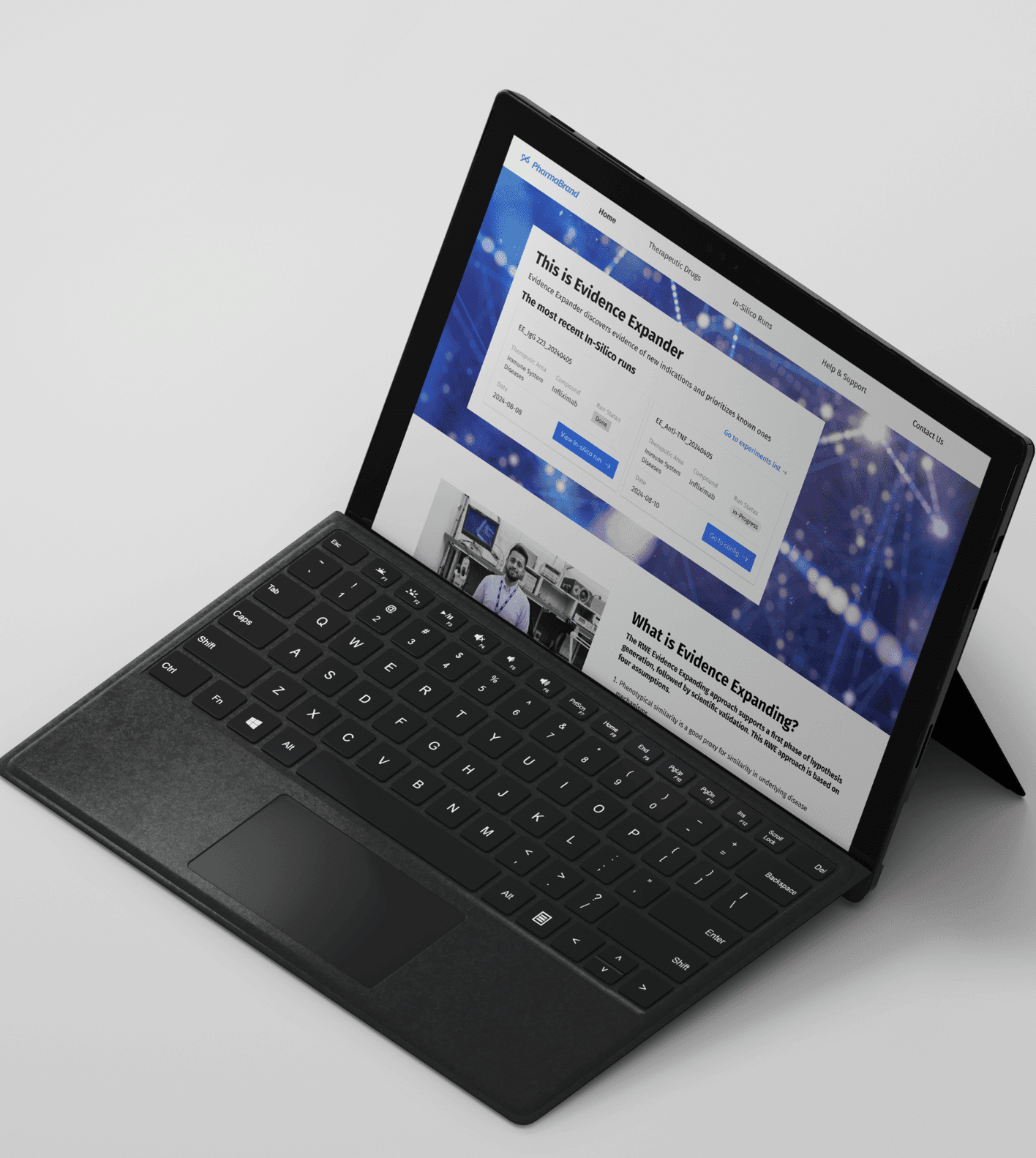
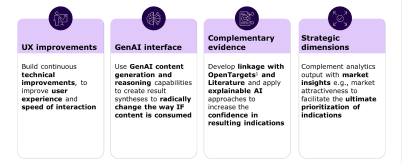
Building ‘Evidence Expander’ to accelerate newer drugs towards the right therapeutic goals
Building industrialised Indication Finding


Building industrialised Indication Finding
Using rich text features within Confluence to conduct expert interviews and document initial takeaways that bring stakeholders along.
Expert interviews within guides with quick takeaways
Understand IF workflow
Before making persona overviews, mapping all stakeholders (core, operational and enablers) and how they interact using the product we build.

Understanding interactions and dependencies between roles
Understand IF workflow
Manual tasks, front-end tasks and back-end engineering tasks conducted pre-industrialisation, all documented with current-state insights and pain-points.
Documenting current workflows and handovers
Understand IF workflow
Reimagining a less manual and more automated front-end workflow and detailing features that address expert user’s needs.
Anchoring product in the future-state journey
Industrialised workflow
Industrialised workflow
Using design file as a foundation for early stage validation of interaction patterns and features.
Iterative deployment via UX/UI prototyping
Industrialised workflow
Industrialised workflow
Translating approved features within defined EPICS and detailed user-stories and sprint planning appropriate effort
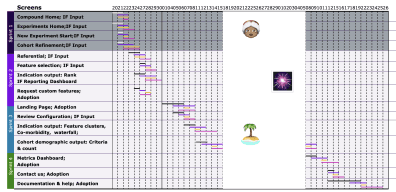
Product owner to translate vision to build
Industrialised workflow
Industrialised workflow
Factoring in the pharmaco asset pipeline to anticipate when the platform will be used most across therapeutic areas.
Designing adoption in line with pipeline
Operationalisation
Operationalisation
Making the small tribe of clinical translators visible within the organisational intranet and showcasing their abilities.

Showcasing capabilities via internal platform
Operationalisation
Operationalisation
Harvesting user and tech requirements into a product roadmap and backlog of stories to be implemented in next quarters.
Documenting product roadmap with new EPICS
Operationalisation
Operationalisation
Using rich text features within Confluence to conduct expert interviews and document initial takeaways that bring stakeholders along.

Expert interviews within guides with quick takeaways
Understand IF workflow
Before making persona overviews, mapping all stakeholders (core, operational and enablers) and how they interact using the product we build.

Understanding interactions and dependencies between roles
Understand IF workflow
Manual tasks, front-end tasks and back-end engineering tasks conducted pre-industrialisation, all documented with current-state insights and pain-points.

Documenting current workflows and handovers
Understand IF workflow
Reimagining a less manual and more automated front-end workflow and detailing features that address expert user’s needs.

Anchoring product in the future-state journey
Industrialised workflow
Industrialised workflow
Using design file as a foundation for early stage validation of interaction patterns and features.

Iterative deployment via UX/UI prototyping
Industrialised workflow
Industrialised workflow
Translating approved features within defined EPICS and detailed user-stories and sprint planning appropriate effort

Product owner to translate vision to build
Industrialised workflow
Industrialised workflow
Factoring in the pharmaco asset pipeline to anticipate when the platform will be used most across therapeutic areas.

Designing adoption in line with pipeline
Operationalisation
Operationalisation
Making the small tribe of clinical translators visible within the organisational intranet and showcasing their abilities.

Showcasing capabilities via internal platform
Operationalisation
Operationalisation
Harvesting user and tech requirements into a product roadmap and backlog of stories to be implemented in next quarters.

Documenting product roadmap with new EPICS
Operationalisation
Operationalisation
Using rich text features within Confluence to conduct expert interviews and document initial takeaways that bring stakeholders along.

Expert interviews within guides with quick takeaways
Understand IF workflow
Before making persona overviews, mapping all stakeholders (core, operational and enablers) and how they interact using the product we build.

Understanding interactions and dependencies between roles
Understand IF workflow
Manual tasks, front-end tasks and back-end engineering tasks conducted pre-industrialisation, all documented with current-state insights and pain-points.

Documenting current workflows and handovers
Understand IF workflow
Reimagining a less manual and more automated front-end workflow and detailing features that address expert user’s needs.

Anchoring product in the future-state journey
Industrialised workflow
Industrialised workflow
Using design file as a foundation for early stage validation of interaction patterns and features.

Iterative deployment via UX/UI prototyping
Industrialised workflow
Industrialised workflow
Translating approved features within defined EPICS and detailed user-stories and sprint planning appropriate effort

Product owner to translate vision to build
Industrialised workflow
Industrialised workflow
Factoring in the pharmaco asset pipeline to anticipate when the platform will be used most across therapeutic areas.

Designing adoption in line with pipeline
Operationalisation
Operationalisation
Making the small tribe of clinical translators visible within the organisational intranet and showcasing their abilities.

Showcasing capabilities via internal platform
Operationalisation
Operationalisation
Harvesting user and tech requirements into a product roadmap and backlog of stories to be implemented in next quarters.

Documenting product roadmap with new EPICS
Operationalisation
Operationalisation
Using rich text features within Confluence to conduct expert interviews and document initial takeaways that bring stakeholders along.

Expert interviews within guides with quick takeaways
Understand IF workflow
Before making persona overviews, mapping all stakeholders (core, operational and enablers) and how they interact using the product we build.

Understanding interactions and dependencies between roles
Understand IF workflow
Manual tasks, front-end tasks and back-end engineering tasks conducted pre-industrialisation, all documented with current-state insights and pain-points.

Documenting current workflows and handovers
Understand IF workflow
Reimagining a less manual and more automated front-end workflow and detailing features that address expert user’s needs.

Anchoring product in the future-state journey
Industrialised workflow
Industrialised workflow
Using design file as a foundation for early stage validation of interaction patterns and features.

Iterative deployment via UX/UI prototyping
Industrialised workflow
Industrialised workflow
Translating approved features within defined EPICS and detailed user-stories and sprint planning appropriate effort

Product owner to translate vision to build
Industrialised workflow
Industrialised workflow
Factoring in the pharmaco asset pipeline to anticipate when the platform will be used most across therapeutic areas.

Designing adoption in line with pipeline
Operationalisation
Operationalisation
Making the small tribe of clinical translators visible within the organisational intranet and showcasing their abilities.

Showcasing capabilities via internal platform
Operationalisation
Operationalisation
Harvesting user and tech requirements into a product roadmap and backlog of stories to be implemented in next quarters.

Documenting product roadmap with new EPICS
Operationalisation
Operationalisation
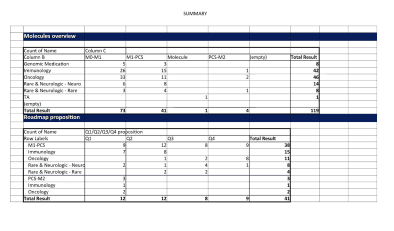
Building ‘Evidence Expander’ to accelerate newer drugs towards the right therapeutic goals
Building ‘Evidence Expander’ to accelerate newer drugs towards the right therapeutic goals
Building ‘Evidence Expander’ to accelerate newer drugs towards the right therapeutic goals
Life-sciences
Clinical translation
Life-Sciences
R&D Large Molecules
Life-sciences
Clinical translation
Life-Sciences
R&D Large Molecules
Life-sciences
Clinical translation
Life-Sciences
R&D Large Molecules
Using rich text features within Confluence to conduct expert interviews and document initial takeaways that bring stakeholders along.

Expert interviews within guides with quick takeaways
Understand IF workflow
Industrialised workflow
Operationalisation
Before making persona overviews, mapping all stakeholders (core, operational and enablers) and how they interact using the product we build.

Understanding interactions and dependencies between roles
Understand IF workflow
Industrialised workflow
Operationalisation
Manual tasks, front-end tasks and back-end engineering tasks conducted pre-industrialisation, all documented with current-state insights and pain-points.

Documenting current workflows and handovers
Understand IF workflow
Industrialised workflow
Operationalisation
Reimagining a less manual and more automated front-end workflow and detailing features that address expert user’s needs.

Anchoring product features in the future-state journey
Understand IF workflow
Industrialised workflow
Operationalisation
Using design file as a foundation for early stage validation of interaction patterns and features.

Iterative deployment via UX/UI prototyping
Understand IF workflow
Industrialised workflow
Operationalisation
Translating approved features within defined EPICS and detailed user-stories and sprint planning appropriate effort

Playing product owner to translate requirements to build
Understand IF workflow
Industrialised workflow
Operationalisation
Factoring in the pharmaco asset pipeline to anticipate when the platform will be used most across therapeutic areas.

Designing platform adoption in line with pipeline
Understand IF workflow
Industrialised workflow
Operationalisation
Making the small tribe of clinical translators visible within the organisational intranet and showcasing their abilities.

Showcasing capabilities via internal platform
Understand IF workflow
Industrialised workflow
Operationalisation
Harvesting user and tech requirements into a product roadmap and backlog of stories to be implemented in next quarters.

Documenting product roadmap with new EPICS
Understand IF workflow
Industrialised workflow
Operationalisation
Using rich text features within Confluence to conduct expert interviews and document initial takeaways that bring stakeholders along.

Expert interviews within guides with quick takeaways
Understand IF workflow
Industrialised workflow
Operationalisation
Before making persona overviews, mapping all stakeholders (core, operational and enablers) and how they interact using the product we build.

Understanding interactions and dependencies between roles
Understand IF workflow
Industrialised workflow
Operationalisation
Manual tasks, front-end tasks and back-end engineering tasks conducted pre-industrialisation, all documented with current-state insights and pain-points.

Documenting current workflows and handovers
Understand IF workflow
Industrialised workflow
Operationalisation
Reimagining a less manual and more automated front-end workflow and detailing features that address expert user’s needs.

Anchoring product features in the future-state journey
Understand IF workflow
Industrialised workflow
Operationalisation
Using design file as a foundation for early stage validation of interaction patterns and features.

Iterative deployment via UX/UI prototyping
Understand IF workflow
Industrialised workflow
Operationalisation
Translating approved features within defined EPICS and detailed user-stories and sprint planning appropriate effort

Playing product owner to translate requirements to build
Understand IF workflow
Industrialised workflow
Operationalisation
Factoring in the pharmaco asset pipeline to anticipate when the platform will be used most across therapeutic areas.

Designing platform adoption in line with pipeline
Understand IF workflow
Industrialised workflow
Operationalisation
Making the small tribe of clinical translators visible within the organisational intranet and showcasing their abilities.

Showcasing capabilities via internal platform
Understand IF workflow
Industrialised workflow
Operationalisation
Harvesting user and tech requirements into a product roadmap and backlog of stories to be implemented in next quarters.

Documenting product roadmap with new EPICS
Understand IF workflow
Industrialised workflow
Operationalisation
Using rich text features within Confluence to conduct expert interviews and document initial takeaways that bring stakeholders along.

Expert interviews within guides with quick takeaways
Understand IF workflow
Industrialised workflow
Operationalisation
Before making persona overviews, mapping all stakeholders (core, operational and enablers) and how they interact using the product we build.

Understanding interactions and dependencies between roles
Understand IF workflow
Industrialised workflow
Operationalisation
Manual tasks, front-end tasks and back-end engineering tasks conducted pre-industrialisation, all documented with current-state insights and pain-points.

Documenting current workflows and handovers
Understand IF workflow
Industrialised workflow
Operationalisation
Reimagining a less manual and more automated front-end workflow and detailing features that address expert user’s needs.

Anchoring product features in the future-state journey
Understand IF workflow
Industrialised workflow
Operationalisation
Using design file as a foundation for early stage validation of interaction patterns and features.

Iterative deployment via UX/UI prototyping
Understand IF workflow
Industrialised workflow
Operationalisation
Translating approved features within defined EPICS and detailed user-stories and sprint planning appropriate effort

Playing product owner to translate requirements to build
Understand IF workflow
Industrialised workflow
Operationalisation
Factoring in the pharmaco asset pipeline to anticipate when the platform will be used most across therapeutic areas.

Designing platform adoption in line with pipeline
Understand IF workflow
Industrialised workflow
Operationalisation
Making the small tribe of clinical translators visible within the organisational intranet and showcasing their abilities.

Showcasing capabilities via internal platform
Understand IF workflow
Industrialised workflow
Operationalisation
Harvesting user and tech requirements into a product roadmap and backlog of stories to be implemented in next quarters.

Documenting product roadmap with new EPICS
Understand IF workflow
Industrialised workflow
Operationalisation
Using rich text features within Confluence to conduct expert interviews and document initial takeaways that bring stakeholders along.

Expert interviews within guides with quick takeaways
Understand IF workflow
Industrialised workflow
Operationalisation
Before making persona overviews, mapping all stakeholders (core, operational and enablers) and how they interact using the product we build.

Understanding interactions and dependencies between roles
Understand IF workflow
Industrialised workflow
Operationalisation
Manual tasks, front-end tasks and back-end engineering tasks conducted pre-industrialisation, all documented with current-state insights and pain-points.

Documenting current workflows and handovers
Understand IF workflow
Industrialised workflow
Operationalisation
Reimagining a less manual and more automated front-end workflow and detailing features that address expert user’s needs.

Anchoring product features in the future-state journey
Understand IF workflow
Industrialised workflow
Operationalisation
Using design file as a foundation for early stage validation of interaction patterns and features.

Iterative deployment via UX/UI prototyping
Understand IF workflow
Industrialised workflow
Operationalisation
Translating approved features within defined EPICS and detailed user-stories and sprint planning appropriate effort

Playing product owner to translate requirements to build
Understand IF workflow
Industrialised workflow
Operationalisation
Factoring in the pharmaco asset pipeline to anticipate when the platform will be used most across therapeutic areas.

Designing platform adoption in line with pipeline
Understand IF workflow
Industrialised workflow
Operationalisation
Making the small tribe of clinical translators visible within the organisational intranet and showcasing their abilities.

Showcasing capabilities via internal platform
Understand IF workflow
Industrialised workflow
Operationalisation
Harvesting user and tech requirements into a product roadmap and backlog of stories to be implemented in next quarters.

Documenting product roadmap with new EPICS
Understand IF workflow
Industrialised workflow
Operationalisation
